Process Validation Including Software | MDV-Solve
Process Validation
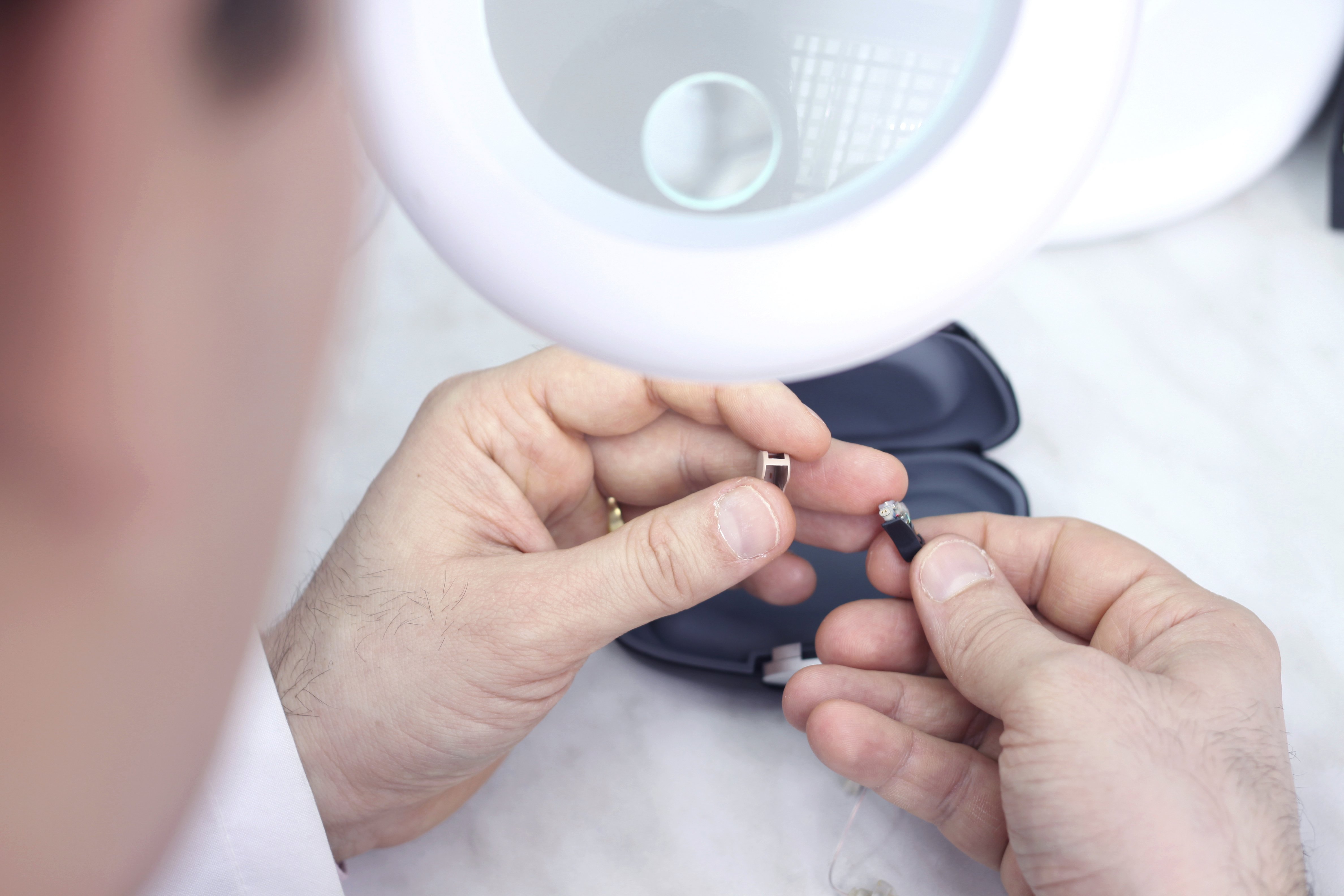
Process validation establishes scientific evidence that a process is capable of consistently delivering quality product. We guide you through IQ, OQ, and PQ.
Services offered:
- Master Validation Plan
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Software validation (per IEC 62304 for medical device software)
Related Services
Clinical Evidence — IVDR
Under IVDR Article 56 - clinical data and performance evaluation results for device safety assessment.
+ Learn MoreThe New EU IVD Regulation (IVDR)
The In Vitro Diagnostic Regulation stipulates all requirements IVD manufacturers must comply with.
+ Learn MoreGood Manufacturing Practice (cGMP)
Establish and implement an effective tailored cGMP system in line with requirements.
+ Learn More